Effective immediately masking is required for everyone when present on all inpatient units, in the Emergency Department (ED), the Urgent Care Centre (UCC), and the Children’s Outpatient Centre (COPC).
Kingston General Hospital, (KGH) the first hospital in Canada to complete a blue light cystoscopy outside of a clinical trial, will soon begin offering the procedure on regular basis to improve the quality of life for patients living with bladder cancer in Southeastern Ontario.
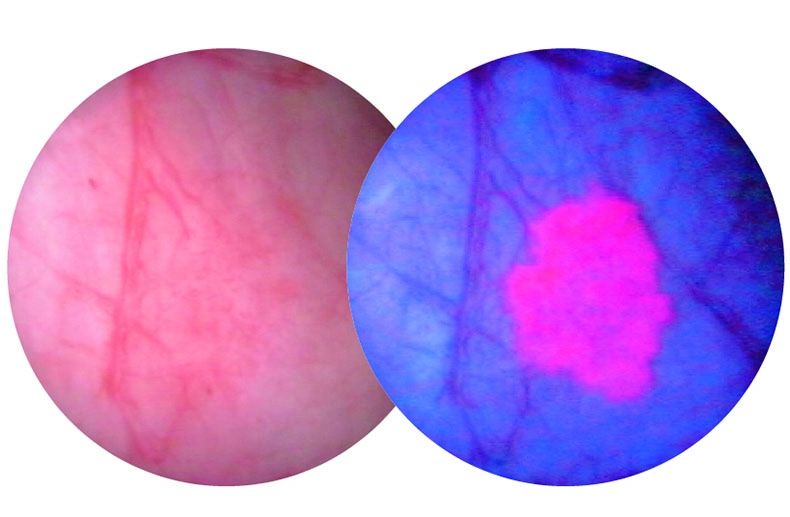
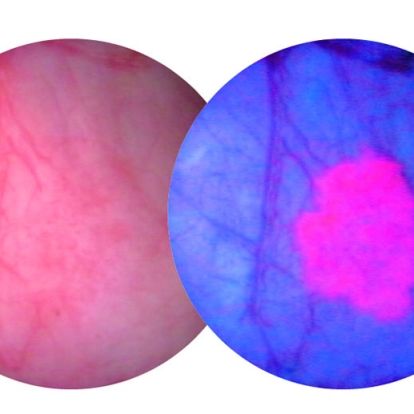
The procedure combines a diagnostic agent known as Cysview along with a scope that detects blue light. When used together, cancerous tissue appears bright pink and is much easier for physicians to see and remove.
In the past, a white light scope was the standard tool used during cystoscopy procedures. When using white light, small tumours could be difficult to see, making it necessary for a patient to undergo multiple follow-up procedures to remove them once they had grown large enough to detect.
“Patients with bladder cancer commonly develop many small tumours rather than one larger one,” says KGH Urologist and Head of Urology at Queen’s University Dr. Robert Siemens. “Now that we can more easily see them, we can remove them more completely during the first procedure.”
To complete the blue light cystoscopy, the Cysview diagnostic agent is given to the patient one hour before the surgery begins so that it can be absorbed by the cancerous tissue. The blue light scope is then inserted into the patient’s urethra and the tumours are removed. The procedure takes about an hour to complete.
“Bladder cancer has a high rate of recurrence, which means that they often grow back, so it’s not uncommon for patients to undergo cystoscopies on a regular basis to check and remove any recurrences,” says Dr. Siemens. “By using this new technique we can now find and remove the smallest tumours, which reduces the number of times a patient must undergo surgery. This represents a significant benefit for the patient.”
The technique has been used in Europe since 2006, but it has taken a number of years to be vetted through clinical trials here in Canada. The Cysview diagnostic agent was recently approved for use by Health Canada.
"The approval by Health Canada and the introduction of Cysview in Canada is a significant and exciting development which will improve bladder cancer diagnosis and treatment," says Ken Bagshaw, bladder cancer patient and Chair of Bladder Cancer Canada. "After many years without advances, we are thrilled that Kingston General Hospital is taking an early lead as one of the first sites to offer Cysview cystoscopy in this country.”
KGH performed its first pilot cases in August 2016, becoming the first hospital in Canada to do so outside of a clinical trial. The hospital has now purchased the equipment and will begin to offer the service on a regular basis for patients with bladder cancer.
“Most of these low risk bladder tumours do not have mortality rates as high as some other cancers, but they are common with an estimated 8,000 people diagnosed in Canada each year,” says Siemens. “By fully adopting this approach we will be able to improve the quality of life for many patients in Southeastern Ontario.”
"One of our hospital’s key strategic goals is that we position ourselves to be national leaders in complex-acute and specialty care. We are so pleased to support our clinical teams by providing funding for new technology so that they may implement innovative approaches to care such as the blue light cystoscopy procedure," says Jim Flett, Interim President & Chief Executive Officer, Kingston General Hospital
This is not the first time that KGH and Queen’s University have been at the forefront in the treatment of bladder cancer. In the 1970’s Dr. Alvaro Morales discovered that a vaccine commonly used to treat Tuberculosis could also be used to reduce the number and frequency of recurrent tumours, as well as to prevent the disease from progressing. It’s a treatment regimen still in use today, earning Kingston a reputation as a leader in the treatment of bladder cancer.
Gallery
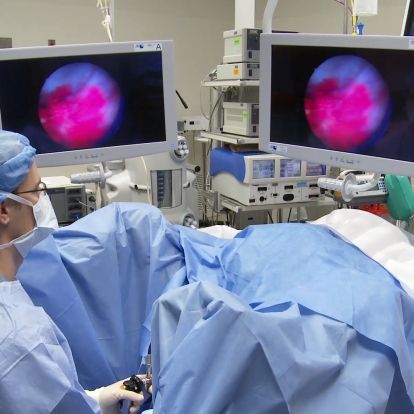
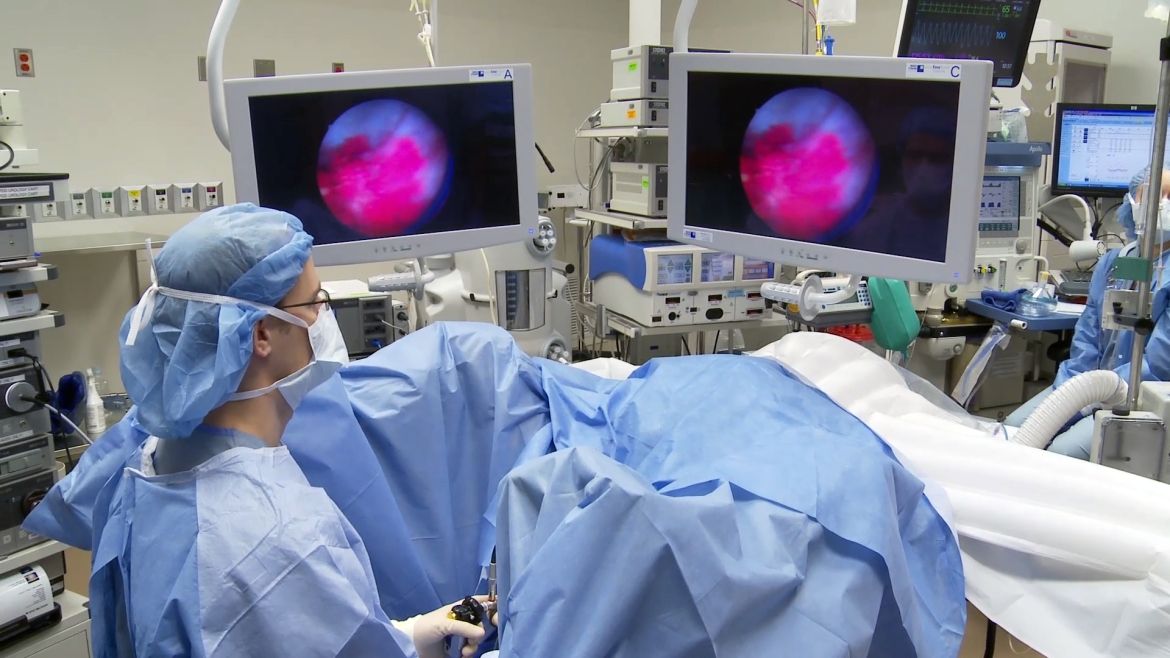
A bladder tumour as seen through a blue light scope.
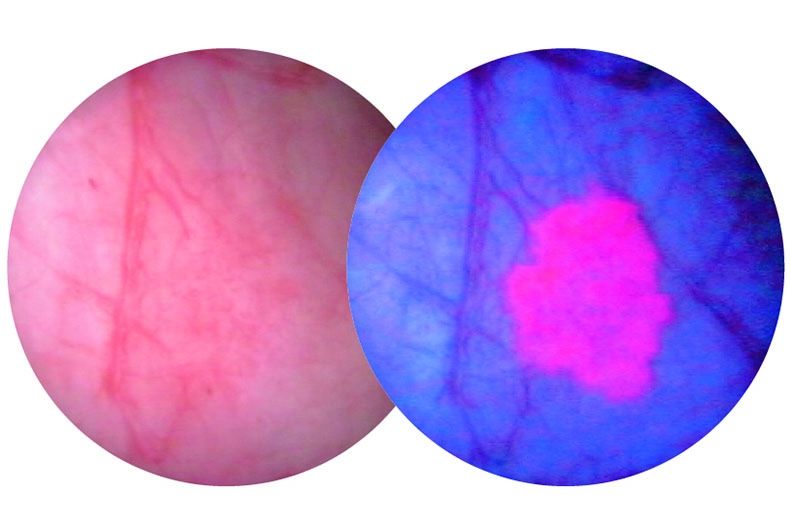
A bladder tumour as seen through a white light scope (L) and a blue light scope (R)



